sk life science xcopri
I am proud and excited to join the talented team at Carmell said Mr. Thats what the company SK Life Sciences one-of-a-kind completely immersive virtual experience has done through its brand Xcopri.

Xcopri Cenobamate Tablets Cv Treatment Support Skl Navigator
Il sagit dun ASM approuvé par la FDA pour le.

. Xcopri는 sk바이오팜이 신약 후보물질 발굴부터 미국 식품의약국fda 허가까지 전 과정을 직접 진행하여 독자적으로 개발한 치료제 입니다. 1 For patients with mild or moderate hepatic impairment the maximum recommended dosage is 200 mg once daily. Selling or giving away XCOPRI may harm others and is against the law.
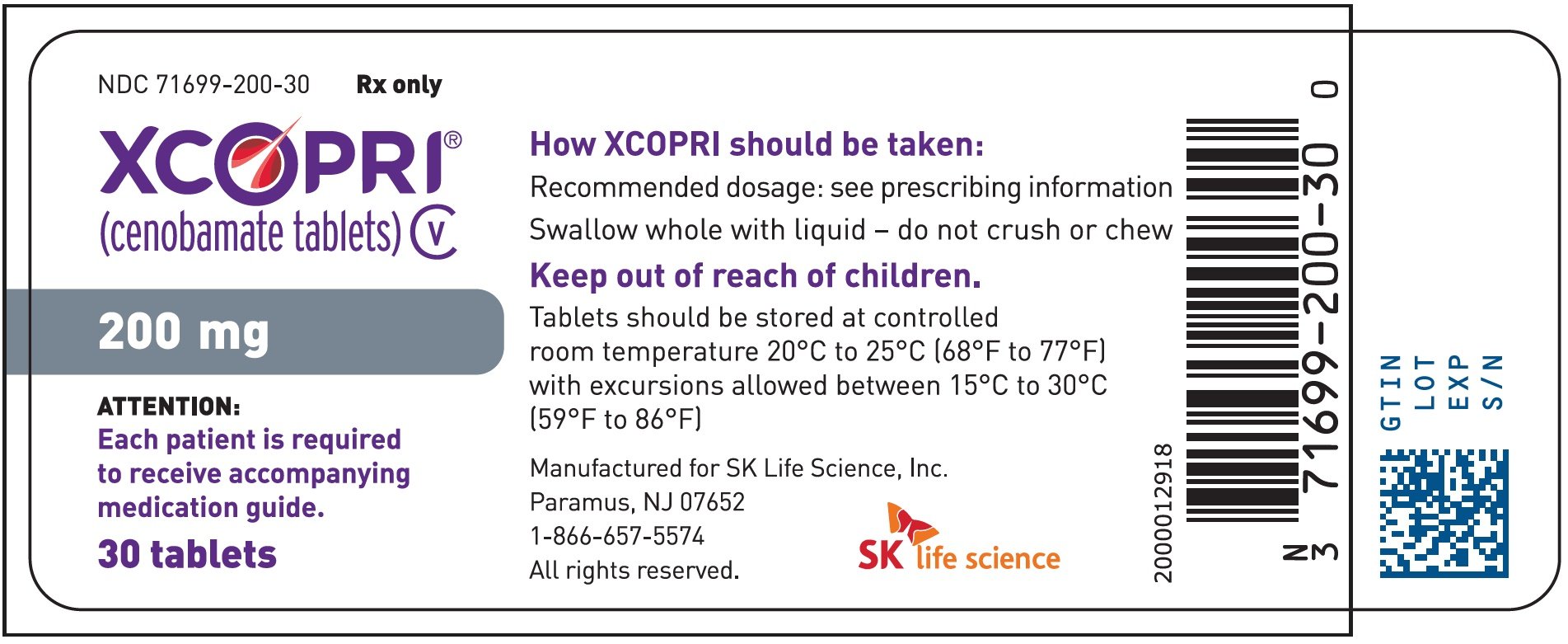
XCOPRI use is not recommended in endstage renal disease. XCOPRI can be prescribed as monotherapy or adjunctive therapy 1. The campaign is called Step into their shoes.
Sebby led the successful US launch of the companys first product XCOPRI in May 2020. If needed based on clinical response and tolerability dosage may be increased above 200 mgday by increments of 50 mgday every 2 weeks to a maximum of 400 mgday. XCOPRI use is not recommended in patients with severe hepatic impairment.
2020년 5월 미국에 출시한 세노바메이트미국 제품명. Le cénobamate a été découvert et développé par SK Biopharmaceuticals et SK life science. And was launched at the American Epilepsy Society AES annual meeting to bring prescribers into the lives of patients and start a discussion on teh company.
Keep XCOPRI in a safe place to prevent misuse and abuse. The maximum recommended daily dose is 200 mg for patients with mild or moderate hepatic impairment. Dosage reduction of XCOPRI may be considered in patients with mild to moderate and severe renal impairment.
XCOPRI is a prescription medicine used to treat partial-onset seizures in adults 18 years of age and older. 세노바메이트 xcopri 미국 제품 사이트로 이동. XCOPRI is a federally controlled substance CV because it can be abused or lead to dependence.
Sebby also played a supportive role in one of the largest biotech IPOs in South Korea 880 million raise during the summer of 2020.

Fda Approves Xcopri Cenobamate Tablets An Anti Epileptic Drug Aed From Sk

Fda Approves Xcopri Cenobamate Tablets An Anti Epileptic Drug Aed From Sk

Sk Life Science To Present Latest Cenobamate Data At The American Academy Of Neurology 2021 Virtual Annual Meeting

Fda Approves Xcopri Cenobamate Tablets An Anti Epileptic Drug Aed From Sk

Sk Biopharmaceuticals Anti Epileptic Med Cenobamate Launched In Us Pulse By Maeil Business News Korea

Xcopri Cenobamate Tablets Cv Treatment Support Skl Navigator

Xcopri Now Available For Partial Onset Seizures In Adults Mpr
Sk Biopharm Records 140 Billion In Q1 Sales Pharma 기사본문 Kbr

Rx Item Xcopri Cenobamate 50mg 30 Tablets By Sk Life Science

Xcopri Fda Prescribing Information Side Effects And Uses

Sk Life Science To Present Latest Xcopri Cenobamate Tablets Cv Data At The American Academy Of Neurology 2022 Annual Meeting

Xcopri Epilepsy Downloadable Resources Hcp

2 Ads For 2 Audiences Sk Life Targets Docs And Patients In Seizure Drug Campaign Fierce Pharma

Xcopri Cenobamate Tablets Cv Treatment Support Skl Navigator

Big Korean Corporate Names Enlarge Footprints In Bio Market Pulse By Maeil Business News Korea

Sk Bio Gains Fda Approval For Xcopri Contract Pharma

Dtw Research Inc On Twitter Xcopri Is Approved For The Treatment Of Partial Onset Seizures In Adult Patients For More Information Https T Co I6vkaqy2yj Skbiopharmaceuticals Sklifescience Xcopri Cenobamate Antiepilepticdrug Aed